Competent Authority, Notified Body, ISO Registrar: How Each Role Functions in the Medical Device Industry

Which EU Notified Bodies Have Been “Designated” Under the MDR 2017/745 and IVDR 2017/746? – Oriel STAT A MATRIX – ELIQUENT Life Sciences Blog
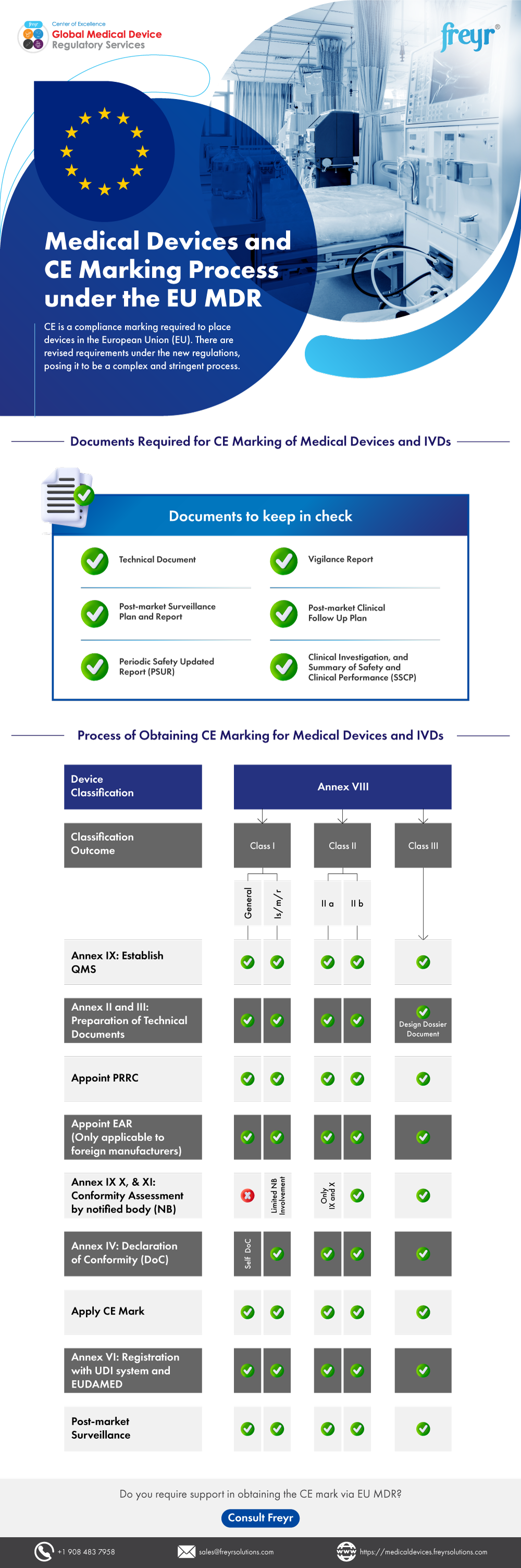
Medical Devices and CE Marking Process under the EU MDR | Freyr - Global Regulatory Solutions and Services Company

EU Finalizes New Medical Device Regulations (MDR) which update the regulatory framework for the marketing of devices and IVDs in Europe – Catchtrial




%20On%20EU%20Notified%20Bodies.jpg)